Cooper Union and Mount Sinai Collab on Recent Publication in Advanced Healthcare Materials
POSTED ON: September 15, 2021
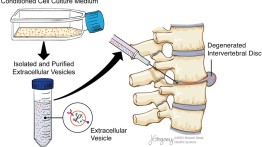
Image of one potential workflow strategy of EV therapy for IVDD.
Jennifer Weiser, assistant professor of chemical engineering, and her graduate student, Keti Vaso ChE’19 ME ’21, have collaborated with Dr. James Iatridis of the Department of Orthopaedics at the Icahn School of Medicine at Mount Sinai (ISMMS) on a recent publication in Advanced Healthcare Materials. The work was done alongside Dr. Iatridis’ recently graduated doctoral student and Cooper alumnus, Tyler DiStefano ME’15, as an introduction into the cooperative research occurring between Dr. Weiser and Dr. Iatridis at the two institutions.
The publication, “Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations,” is a guide to the field of harnessing biologically derived extracellular vesicles (EVs) as a cell-free drug treatment option for degenerative diseases of the spine, specifically intervertebral disc degeneration (IVDD).
EVs are lipid bound secretory packages that nearly all cells release and are involved in numerous physiological and pathological processes. This publication lays out a comprehensive overview of EV biogenesis, cellular isolation and characterization techniques, various in vitro and in vivo work done to date, and methods to assess their effectiveness in reducing painful IVDD. Furthermore, the work provides an overview of the regulatory approval process through the FDA to advance the translation of EVs from the benchtop to the clinic as a new therapeutic for IVDD.
This work was first facilitated by the interactions fostered through the Cooper Union-Mount Sinai collaborative exchange and annual symposium. Additional co-authors include current ISMMS medical student George Danias and recent ISMMS Master’s student Henry Chionuma.
You can view the publication here.
ABSTRACT
Emergent approaches in regenerative medicine look toward the use of extracellular vesicles (EVs) as a next-generation treatment strategy for intervertebral disc (IVD) degeneration (IVDD) because of their ability to attenuate chronic inflammation, reduce apoptosis, and stimulate proliferation in a number of tissue systems. Yet, there are no Food and Drug Administration (FDA)-approved EV therapeutics in the market with an indication for IVDD, which motivates this article to review the current state of the field and provide an IVD-specific framework to assess its efficacy. In this systematic review, 29 preclinical studies that investigate EVs in relation to the IVD are identified, and additionally, the regulatory approval process is reviewed in an effort to accelerate emerging EV-based therapeutics toward FDA submission and timeline-to-market. The majority of studies focus on nucleus pulposus responses to EV treatment, where the main findings show that stem cell-derived EVs can decelerate the progression of IVDD on the molecular, cellular, and organ level. The findings also highlight the importance of the EV parent cell's pathophysiological and differentiation state, which affects downstream treatment responses and therapeutic outcomes. This systematic review substantiates the use of EVs as a promising cell-free strategy to treat IVDD and enhance endogenous repair.




